

(Gerd. & Trappe) R.T. Almeida & N.C. Schenck
 |
 |
 |
 |
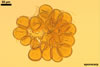
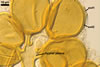
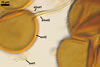
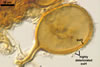
SPORES occur in sporocarps in the soil. Sporocarps pale yellow (3A3) to light brown (6D8); globose; 200-260 µm diam or ovoid; 100-200 x 190-360 µm; without a peridium; with 3 to 18 spores. Spores pale yellow (3A3) to light brown (6D8); globose to subglobose; (40.0-)52.0(-70.0) µm diam; or ovoid to prolate; 35-45 x 50-70 µm; with a single subtending hypha; developed from a thick-walled, inflated hypha; spores arranged in a hemispherical layer in young sporocarps or radially to form a blackberry-like sporocarp when mature.
 |
 |
 |
 |
 |
In PVLG |
||||
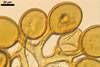
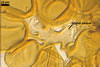
Layer 1 evanescent, hyaline, (0.3-)0.5(-0.8) µm thick before disintegration, closely adherent to layer 2.
Layer 2 laminate, pale yellow (3A3) to light brown (6D8), (2.7-)3.1(-3.7) µm thick.
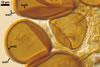
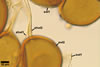
SUBTENDING HYPHA pale yellow (3A3) to light brown (6D8); straight to recurvate; funnel-shaped, sometimes cylindrical or constricted; (8.8-)10.7(-14.2) µm wide at the spore base.
 |
 |
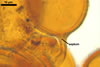 |
In PVLG |
||
Pore (1.5-)2.2(-2.9) µm wide, occluded by a septum, ca. 2.0 µm wide, continuous with the innermost lamina of wall layer 2, and occasionally by thickening of spore wall layer 2.
GERMINATION. Not observed.
MYCORRHIZAE. Many attempts to initiate sporulation of Gl. rubiforme in both trap and one-species cultures failed. No report exists of the properties of mycorrhizae of this fungus.
DISTRIBUTION. In Poland, Gl. rubiforme has been found in many soils coming from different physiographic regions (Błaszkowski et al. 1998, 2002; Tadych and Błaszkowski 2000a, b). The soils represented both cultivated and uncultivated sites, the letter including forests, heaps, maritime and inland dunes.
Glomus rubiforme probably is a widely distributed fungus in the world. Its occurrence has been reported in Florida, Michigan, New York, Oregon and Washington of the U.S.A. (Gerdemann and Trappe 1974; Miller et al. 1985; Nicolson and Schenck 1979), in Canada (Dalpé 1989; Dalpé et al. 1986; Hamel et al. 1994), Brazil (Grandi and Trufem 1991; Grandi et al. 1987) and Colombia (Sieverding 1989), England and Wales (Mosse and Bowen 1968), Cameroon (Musoko et al. 1994), India (Bhattacharjee et al. 1980; Ragupathy and Mahadevan 1993), Taiwan (Wu 1993; Wu and Chen 1986), and New Zealand (Hall 1977; Johnson 1977; Mosse and Bowen 1968).
NOTES. The distinctive features of Gl. rubiforme are its sporocarps with relatively small, coloured spores originated from a centrally positioned hyphal plexus (Almedia and Schenck 1990; Błaszkowski et al. 1998; Wu 1993). When young, the sporocarps resemble a hemispherical layer. At times, they convert into blackberry-like structures due to the formation of subsequent spores radially developing. Single sporocarps sometimes are connected with hyphae in larger aggregates. The sporocarps are never enveloped in a peridium. The hyphal plexus of sporocarps consists of an inflated, thick-walled cell. Hyphal branches erect from this cell and swell at their tip, forming spores. At first, these branches are thin-walled. At times, they become thicker and more rigid due to the synthesis of further sublayers in the laminate spore wall 2.
The single wall of Gl. rubiforme spores is composed of two layers: a thin, sloughing, hyaline outer layer and a thicker, coloured, laminated inner layer. The outer spore wall layer is rarely present, especially in field-collected spores. None of these layers stains in Melzer’s reagent.
The subtending hypha of Gl. rubiforme usually is funnel-shaped, although cylindrical or constricted subtending hyphae were also found in specimens examined by the author of this website. The subtending hyphal wall highly thickens with spore age due to the addition of sublayers to the inner surface of its laminate layer 2. This causes mature spores to usually have a subtending hypha with a narrow lumen connecting the spore inside with the hypha. The lumen in most mature spores is occluded by 1-3 thick septa. Young sporocarps usually contain spores with subtending hyphae without septa.
Species of arbuscular fungi most similar in appearance to Gl. rubiforme are Gl. ambisporum G.S. Sm. & N.C. Schenck, Gl. heterosporum G.S. Sm. & N.C. Schenck, and Gl. taiwanense (C.G. Wu & Z.C. Chen) R.T. Almeida & N.C. Schenck. All the fungi produce spores in globular sporocarps. However, spores of Gl. ambisporum compared with those of Gl. rubiforme are larger [85-157 µm diam vs. 27-125 x 29-87(-110) µm (Almeida and Schenck 1990); 27.5-60.0 x 37.5-87.5 µm (Wu 1993); (40.0-)52.0(-70.0) µm (Błaszkowski, pers. observ.)] and have a thicker wall [6-18 µm vs. 3.0-7.6, up to 13.5 µm at spore base (Almeida and Schenck 1990); 1.5-6.0(-8.5) µm (Wu 1993); (2.7-)3.1(-3.7) µm (Błaszkowski pers. observ.)] with a reticulate outermost layer (vs. the smooth layer in Gl. rubiforme). Glomus heterosporum also forms larger spores (99-206 x 61-201 µm) than Gl. rubiforme; they frequently possess many subtending hyphae (a single subtending hypha in Gl. rubiforme). Additionally, both Gl. ambisporum and Gl. heterosporum are dimorphic fungi, whereas Gl. rubiforme forms one type of spores.
Although spores of Gl. rubiforme are in the same size range as those of Gl. taiwanense, the plexal hypha of the former fungus is a broad, thick-walled cell around which spores are produced, and that of the latter species is formed by fusion of more than one monohyphal stalk (Wu 1993).
REFERENCES
Almeida R. T., Schenck N. C. 1990. A revision of the genus Sclerocystis (Glomaceae, Glomales). Mycologia 82, 703-714.
Bhattacharjee M., Mukerji K. G., Misra S. 1980. Studies on Indian Endogonaceae. III. Further records. Acta Bot. Indica 8, 99-102.
Błaszkowski J., Madej T., Tadych M. 1998. Glomus rubiforme (Glomales, Zygomycetes), an arbuscular mycorrhizal fungus new to the mycota of Poland. Acta Mycol. 33, 255-263.
Błaszkowski J., Tadych M., Madej T. 2002. Arbuscular mycorrhizal fungi (Glomales, Zygomycota) of the Bledowska Desert, Poland. Acta Soc. Bot. Pol. 71, 71-85.
Dalpé Y. 1989. Inventaire et repartition de la flore endomycorhizienne de dunes et de rivages maritimes du Quebec, du Nouveau-Brunswick et de la Nouvelle-Ecosse. Naturaliste can. (Rev. Ecol. Syst.) 116, 219-236.
Dalpé Y., Granger R. L., Furlan V. 1986. Abondance relative et diversite des Endogonacees dans un sol de verger du Quebec. Canad. J. Bot. 64, 912-917.
Gerdemann J. W., Trappe J. M. 1974. The Endogonaceae in the Pacific Northwest. Myc. Memoir 5, 1-76.
Grandi R. A. P., Trufem S. F. B. 1991. Fungos micorrizocos vesiculo-arbusculares em Marantaceae cultivadas no Instituto de Botânica, Säo Paulo, SP. Revta Brasil. Bot. 14, 89-95.
Grandi R. A. P., Trufem S. F. B., Komesu S. T. 1987. Fungos micorrizocos em quatro espécies de Marantaceae. P. I. In: Reuniâo Brasileira sobre Micorrhizas, Säo Paulo, SP.
Hall I. R. 1977. Species and mycorrhizal infections of New Zealand Endogonaceae. Trans. Br. Mycol. Soc. 68, 341-356.
Hamel C., Dalpé Y., Lapierre C., Simard R. R., Smith D. I. 1994. Composition of the vesicular-arbuscular mycorrhizal fungi population in an old meadow as affected by pH, phosphorus and soil disturbance. Agric. Ecosys. Environ. 49, 223-231.
Johnson P. N. 1977. Mycorrhizal Endogonaceae in a New Zealand forest. New Phytol. 78, 161-170.
Miller D. D., Domoto P. A., Walker C. 1985. Mycorrhizal fungi at eighteen apple rootstock plantings in the United States. New Phytol. 100, 379-391.
Mosse B., Bowen G. D. 1968. The distribution of Endogone spores in some Australian and New Zealand soils, and in an experimental field soil at Rothamsted. Trans. Br. Mycol. Soc. 51, 485-492.
Musoko M., Last F. T., Mason P. A. 1994. Populations of spores of vesicular-arbuscular mycorrhizal fungi in undisturbed soils of secondary semideciduous moist tropical forest in Cameroon. Forest Ecol. Management 63, 359-377.
Nicolson T. H., Schenck N. C. 1979. Endogonaceous mycorrhizal endophytes in Florida. Mycologia 71, 178-198.
Ragupathy S., Mahadevan A. 1993. Distribution of vesicular-arbuscular mycorrhizae in the plants and rhizosphere soils of the tropical plains, Tamil Nadu, India. Mycorrhiza 3,: 123-136.
Sieverding E. 1989. Ecology of VAM fungi in tropical agrosystems. Agric., Ecosyst. and Environment. 29, 369-390.
Tadych M., Błaszkowski J. 2000a. Arbuscular fungi and mycorrhizae (Glomales) of the Slowinski National Park, Poland. Mycotaxon 74, 463-483.
Tadych M., Błaszkowski J. 2000b. Arbuscular mycorrhizal fungi of the Brda river valley in the Tuchola Forests. Acta Mycol. 35, 3-23.
Wu C.-G. 1993. Glomales of Taiwan: IV. A monograph of Sclerocystis (Glomaceae). Mycotaxon 49, 327-349.
Wu C.-G., Chen Z. C. 1986. The Endogonaceae of Taiwan. I. A preliminary investigation on Endogonaceae of babmbo vegetation at Chi-Tou areas, Central Taiwan. Taiwania 31, 65-88.